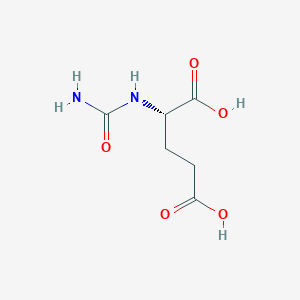
Hyperammonaemia in patients with N-acetylglutamate synthase deficiency
Adult: Initially, 100-250 mg/kg daily given as 2-4 divided doses immediately prior to meals, adjusted thereafter to maintain normal ammonia plasma levels. Maintenance: 10-100 mg/kg daily.




 Sign Out
Sign Out